Online Middle School Chemistry Resource
Updated: 2024-04-26 01:39:40
The ACS Education Division has developed a new middle school chemistry resource, "Middle School Chemistry: Big Ideas about the Very Small." This free, six-chapter resource can serve as either a stand-alone chemistry unit or as a supplement to any middle school science curriculum. "Middle School Chemistry" uses a hands-on inquiry approach along with specially designed molecular model animations, to take students from concrete experiences to an understanding of the world of atoms and molecules. Share this free resource with middle school teachers you know.

 Join Login About Us News Events A Message from the Chair Latest News Calendar of Events Newsletter Archive Membership Join Contact Us Feedback Membership Benefits Membership Benefits PDF The Division Executive Committee Bylaws Frequently Asked Questions History of the Division Awards Earned by the Division indicates you must be a member of the DOC to . access Resources Videos Eminent Organic Chemistsâ Videos Historic Chemistry Videos Past Virtual Symposia NOS Lecture Videos Other Organic Chemistry Videos Literature Organic Chemistry Journals Organic Syntheses Organic Reactions Chemistry Reference Resolver Organic Related RSS Feeds Organic Chemistry Info Organic Chemistry Data Website Links to Organic Chemistry Sources Green Organic Chemistry Organic Solvent Data Synthetic Organic Groups
Join Login About Us News Events A Message from the Chair Latest News Calendar of Events Newsletter Archive Membership Join Contact Us Feedback Membership Benefits Membership Benefits PDF The Division Executive Committee Bylaws Frequently Asked Questions History of the Division Awards Earned by the Division indicates you must be a member of the DOC to . access Resources Videos Eminent Organic Chemistsâ Videos Historic Chemistry Videos Past Virtual Symposia NOS Lecture Videos Other Organic Chemistry Videos Literature Organic Chemistry Journals Organic Syntheses Organic Reactions Chemistry Reference Resolver Organic Related RSS Feeds Organic Chemistry Info Organic Chemistry Data Website Links to Organic Chemistry Sources Green Organic Chemistry Organic Solvent Data Synthetic Organic Groups Encourage your students to use their hands to help them get to grips with complex chemistry concepts
Encourage your students to use their hands to help them get to grips with complex chemistry concepts Thanks to members of the DOC Web team we are excited to announce that Division Members can now access the ORGN abstract separates for the 267th ACS National Meeting to be held March 17–21 in New Orleans (Spring 2024). To access the file, you must be a Division Member. You can download the PDF file …
Read More
The post Abstracts for the Spring 2024 ACS Meeting in New Orleans appeared first on ACS Division of Organic Chemistry.
Thanks to members of the DOC Web team we are excited to announce that Division Members can now access the ORGN abstract separates for the 267th ACS National Meeting to be held March 17–21 in New Orleans (Spring 2024). To access the file, you must be a Division Member. You can download the PDF file …
Read More
The post Abstracts for the Spring 2024 ACS Meeting in New Orleans appeared first on ACS Division of Organic Chemistry.
 Discover how learners use electronegativity to predict the location of dipoleâdipole interactions
Discover how learners use electronegativity to predict the location of dipoleâdipole interactions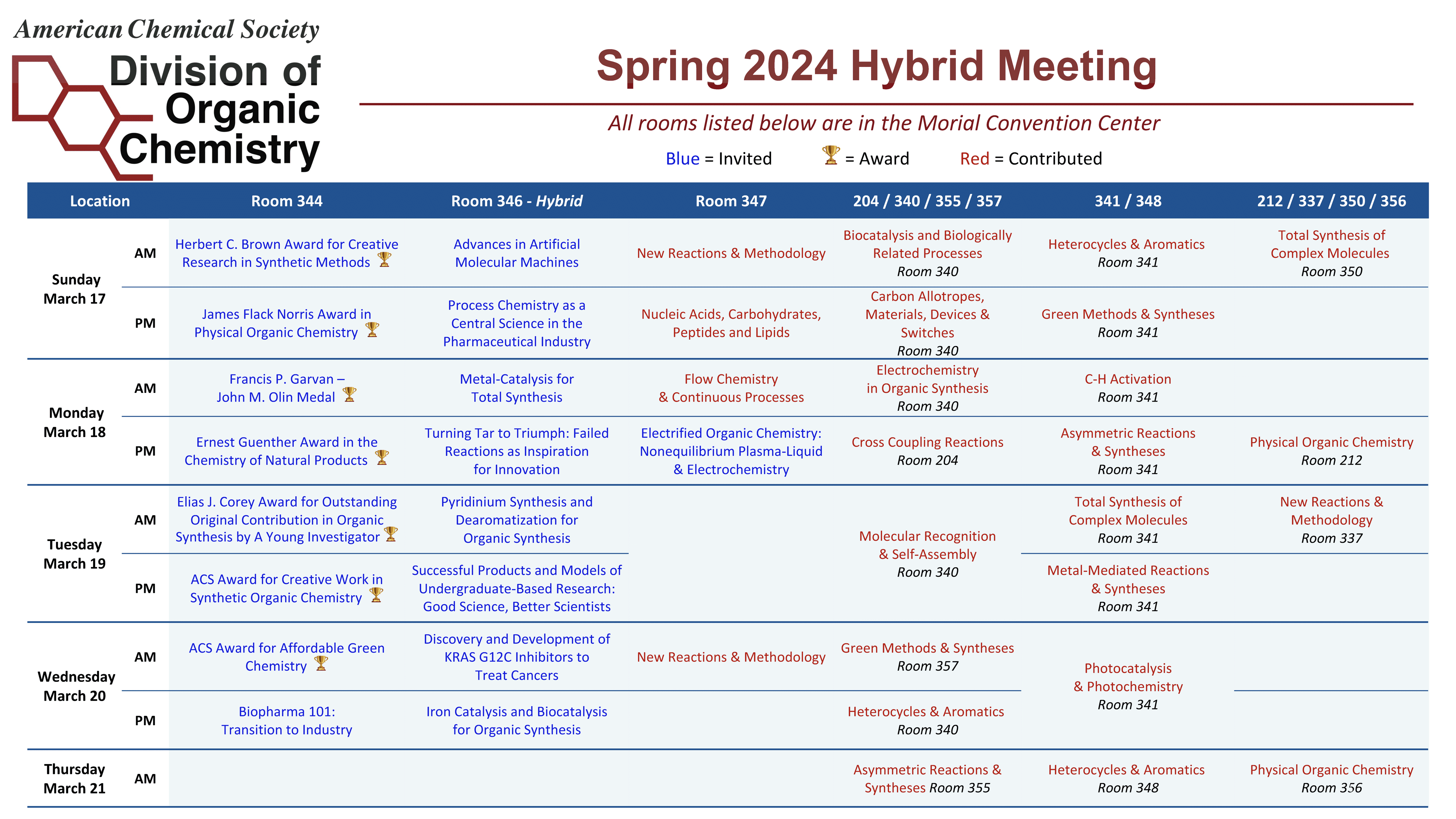 The Organic Division Program Chair, has put together a grid of all the sessions housed within the Organic Division at the upcoming ACS meeting in New Orleans (with rooms included).
The post ORGN Program Grid-Spring 2024 ACS Meeting appeared first on ACS Division of Organic Chemistry.
The Organic Division Program Chair, has put together a grid of all the sessions housed within the Organic Division at the upcoming ACS meeting in New Orleans (with rooms included).
The post ORGN Program Grid-Spring 2024 ACS Meeting appeared first on ACS Division of Organic Chemistry.